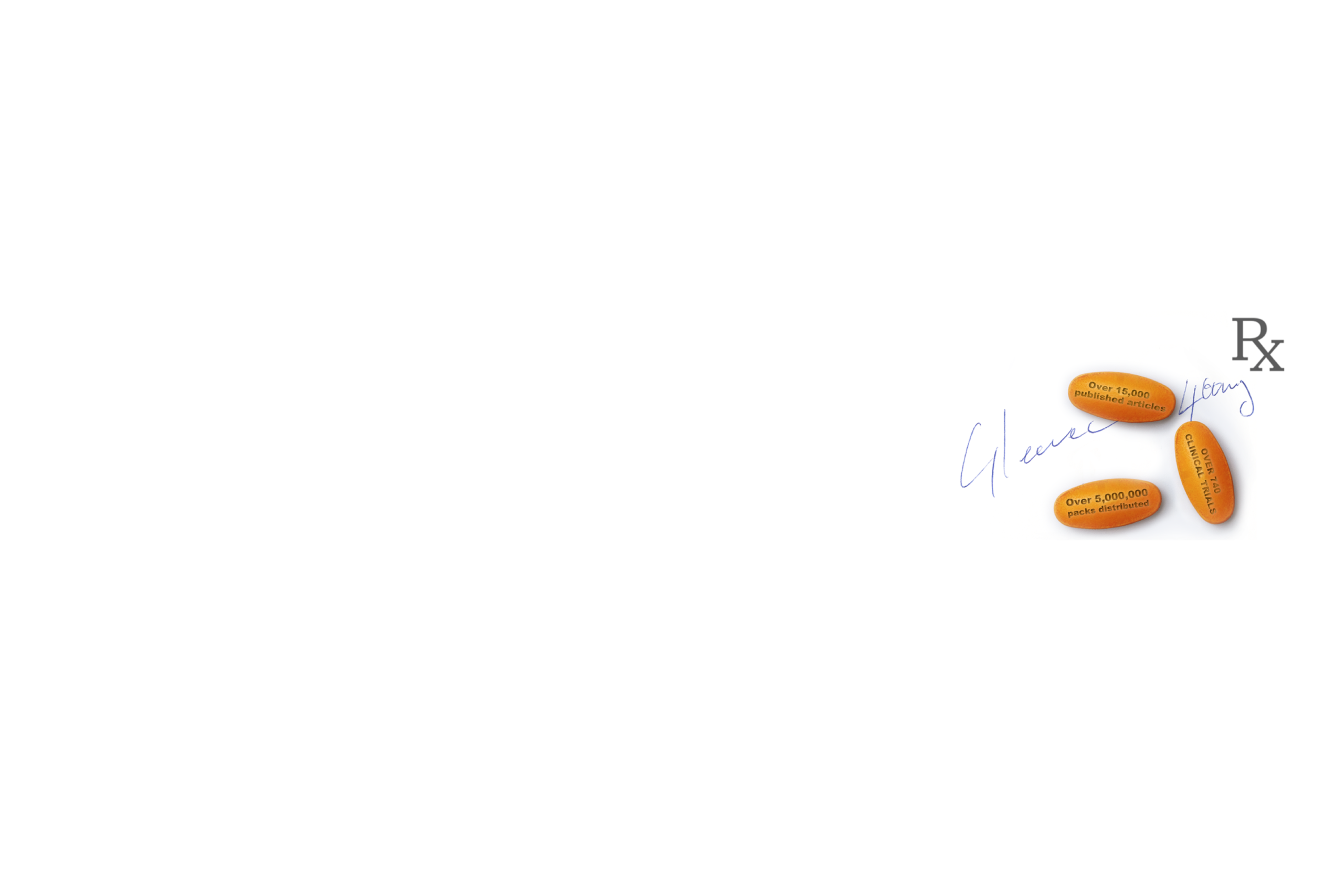GLEEVEC is supported by clinical trial data backed by more than two decades of clinical use.1 First approved by the US Food and Drug Administration in 2001,1 the figures speak for themselves:
Over 15,000 published articles2
Over 740 clinical trials3
Over 5,000,000 packs distributed4
GLEEVEC has a well-established safety profile.